The New Fight Against Cancer
By Alexander Nazaryan
From his fourth-floor window at Tampa’s Moffitt Cancer Center, Robert A. Gatenby can look down to where patients stand waiting for valets to retrieve their cars. They have gone through chemotherapy, biopsies, radiation. They are pale, anxious, resolute. Some will live and some will die: a young woman with short hair, clutching her partner’s hand; an older man, alone. Students from the nearby University of South Florida pop out of patients’ cars. Peppy and dressed in blue vests, these cheerful valets look as if they could be working at a luxury hotel in the tropics. But nobody here is on vacation.
Gatenby says he sometimes sees patients retching after chemotherapy, which reminds the 62-year-old radiologist that his Integrated Mathematical Oncology Department—the only full-scale outfit of its kind in the nation—does not have the luxury of time. Mathematics is not generally known for urgency. Few lives hinge on proof of the twin prime conjecture, but the mathematicians and oncologists Gatenby has assembled in Tampa are trying to tame the chaos of cancer in part through the same differential equations that have tortured so many generations of calculus students. By mathematically modeling cancer, they hope to solve it, to make its movements as predictable as those of a hurricane. The patients down there, fresh from treatment, need shelter from the storm.
Gatenby’s small corner of Moffitt bears little resemblance to a medical center: There are no white-coated doctors frantically rushing to save patients or synthesizing miracle cures deep into the night. You might think you’ve found yourself in a sleepy academic department where abstract ideas are kicked around like a soccer ball on the college green. Which, come to think of it, is actually a pretty accurate description of what goes on in Gatenby’s lab, though not at all a pejorative one. The mathematicians in his employ are convinced that we do not really understand cancer and that, until we do, our finest efforts will be tantamount to swinging swords in utter darkness. As far as these Tampa iconoclasts are concerned, your average cancer doctor is trying to build a jetliner without having grasped aerodynamics: Say, how many wings should we slap on this thing?
A Malicious Green Cloud
We have been fighting the War on Cancer since 1971, when President Richard M. Nixon declared that the “time has come in America when the same kind of concentrated effort that split the atom and took man to the moon should be turned toward conquering this dread disease.” Four decades later, 1,665,540 Americans per year hear the dreaded diagnosis, and about 585,720 die annually from some variety of the disease, according to the American Cancer Society. Smallpox and polio have been cured or largely eradicated, but cancer remains the same scourge it was 4,500 years ago, when the Egyptian doctor Imhotep mused, in what may have been civilization’s first stab at oncology, about how to treat “bulging masses on [the] breast.” Modern oncology makes incremental advances, with a melanoma drug that extends survival by three months passing for a major breakthrough. This is nobody’s fault, but everybody’s problem.
Gatenby is tired of a fight we keep losing. After 30 years, he has come to the uneasy conclusion that cancer is smarter than we are, and will find ways to evade our finest medical weaponry. The weary warrior wants to make peace with cancer’s insurgent cells—though on his own terms, terms that would spare the lives of many more patients. To some within the medical establishment, this might seem preposterous, but Gatenby relishes the role of the outsider.
Gatenby grew up in the Rust Belt town of Erie, Pa., where 12 years of Catholic school instilled in him “an incredible hatred of dogma.” At Princeton University, he studied physics with some of the greatest scientific minds of the 20th century. Figuring he wasn’t fated to join the physics pantheon, Gatenby turned to medicine. But medical school at the University of Pennsylvania was dismayingly similar “to the rote learning of catechism” he remembered from Saint Luke School. It felt like he was “going backwards.”
Whether in the lab, the classroom or the clinic, Western medicine relies on cautious experimentation, its zeal for breakthroughs tempered by the Hippocratic injunction to do no harm. But that can foster a frustrating incrementalism that is itself injurious. David B. Agus, one of the nation’s most prominent oncologists and a professor at the University of Southern California, explains that “you are not rewarded, in general, for taking risk. It’s very scary to do something radically new.”
Gatenby specialized in radiology and, after receiving his medical degree in 1977 and completing a residency, went to work in 1981 for the Fox Chase Cancer Center in Philadelphia. Fox Chase is to cancer research what the Boston Garden was to professional basketball. It was home to David A. Hungerford, one of two researchers responsible for discovering the Philadelphia Chromosome, a major clue to cancer’s birth within the human genome. Among its current éminences grises is Alfred G. Knudson Jr., whose “two-hit” hypothesis holds that cancer is triggered by an unfortunate accumulation of errant genes, harmful outside events (too much sun, too much red meat) or a combination of the two.
The study of genes did not interest Gatenby back then, nor does it interest him now, even though much of medicine is now in the thrall of genomics. Gatenby wanted to discover cancer’s “first principles,” the basic ideas behind the seemingly sudden explosion of cells that want to kill the very body that nourishes them. Sure, you could know the BRCA1 gene better than you know your own mother, but unless you had some insight into why it caused a furiously impervious breast cancer, you were trying to find your way out of a forest by studying the bark of a single tree. Gatenby sought to understand cancer with the same totality that Newton had understood gravity.
As with Newton’s famous laws of motion, mathematics seemed to hold the key. Math had been used to model the weather and financial markets, which like the human body are fickle and incredibly sensitive to outside forces (a run on Greek banks; a low-pressure system moving down from Canada). Gatenby saw no reason the same could not hold true for cancer. He spent a year reading math, which puzzled his colleagues. Then, while visiting the Cloisters museum in upper Manhattan with his family, he took a sheet of stationery and started scratching down equations he thought could get him closer to cancer’s fundamental truths.
“To say they hated it would not do justice,” Gatenby says of the response of his Fox Chase colleagues. Other oncologists told him that “math modeling is for people too lazy to do the experiment” and that “cancer is too complicated to model.” The latter is a refrain that, 30 years later, still dogs Gatenby and his staff at the Integrated Mathematical Oncology Department, which includes five mathematicians with no formal experience in medicine.
Among those five is Sandy Anderson, a young Scotsman who dresses as if he were on the way to a Beck concert. There is a bottle of single malt on his desk. “Of course cancer is complex,” Anderson tells me, brogue rising. “But how can you say it’s too complex? That complexity should be viewed as a challenge that we have to try and tackle. And just because there’s complexity doesn’t mean there aren’t simple rules underlying it.
“What we’d love to do is have everybody’s own little hurricane model for their cancer,” he explains. This is less a metaphor than you may imagine. Anderson shows me computer models of a breast cancer’s growth, the cells spreading like a malicious green cloud across the screen. Different versions of the model show what happens when different treatments are applied: Sometimes the cancer slows, but sometimes it explodes. This seems like an intuitively rational approach to the disease, predicting how it responds to a variety of treatments. But it isn’t common. There are about a dozen drugs for breast cancer approved by the Food and Drug Administration. Depending on which form of the disease is diagnosed and at what stage it’s discovered, there’s a maddening number of viable drug combinations. Best practices exist, but these can be anecdotal, doctors simply doing what they think works. The War on Cancer is fought by competing bands with their own weapons, cancer’s chaos exacerbated by our own dismaying disorder. Anderson would like to provide the onco-soldiers with battlefield maps.
Dr Robert Gatenby, Moffitt Center (man in office with gray hair) Kyoko Hamada for Newsweek
Wrong But Useful
Weather often came up during my time in Tampa, and not only because a wet dreariness lingered in the Florida skies. In 1961, Edward N. Lorenz of the Massachusetts Institute of Technology tried to create computer models for weather, only to stumble into the field of chaos theory. He saw that weather was entirely dependent on initial conditions, so that if he altered his inputs by even a fraction of a percentage, the weather model would fluctuate to an unexpected degree, in unexpected directions. Yet patterns did emerge. This would come to be called “deterministic chaos,” for the way complex adaptive systems—the weather, the global economy, maybe cancer—can both hew to our expectations and routinely subvert them. Sometimes, autumn acts like autumn. But once in a while, in Lorenz’s famous formulation, a butterfly causes a hurricane.
One refrain I heard several times at Moffitt was that “all models are wrong, but some are useful,” a quip by the late mathematician George E.P. Box. A mouse injected with melanoma is only an imperfect model of human cancer; if it weren’t, you wouldn’t be reading this article today, for, as Anderson acidly notes, “We’ve solved cancer in mice a hundred thousand times.” This is a model, too:
If that freaks you out, don’t worry—it freaks out a lot of clinicians. Gatenby and his team are doing the math for them, convinced that their models of cancer strike the right balance between specificity and universality.
The other option is to keep chasing errant genes and trying to snuff them out, but that seems to many like a futile enterprise, sort of like trying to plug a leaking dam with wads of cotton. A tumor that weighs just 10 grams, Gatenby says, contains more cells than there are humans on earth. Nor are those cells a uniform gray mass, as the popular conception of cancer has it. As the tumor grows, different mutations may come to the fore, sort of the way a military assault may deploy infantry and artillery at different times in an attack. The cells of a single cancer differ within a single patient, and the same types of cancers differ from patient to patient. Talking about a prototypical cancer, then, is about as helpful as talking about a prototypical dog.
“It’s almost like it’s an intelligent opponent,” says Donald A. Berry, who heads the biostatistics department at the M.D. Anderson Cancer Center in Houston. “It has many, many paths that it can take.” Mathematics, Berry says, can “provide answers where biology runs into a wall.”
One of those walls is the sheer amount of information cancer researchers would need to map every possible genetic mutation possible for the 200 cancers that can ravage the human body. Researchers have spent $375 million to create the Cancer Genome Atlas, which is based on the screening of 10,000 cancer samples for the responsible genes. Some think that until we’ve sorted through about 100,000 samples, the cancer gene compendium will be woefully incomplete. “It would be crazy not to have the information,” the geneticist Eric S. Lander told The New York Times.
But information brings its own delusions. Gatenby laments the “vast industry that’s developed over molecular data.” He is frustrated by the narrow focus of many of his colleagues. The bookshelves in his office don’t hold the standard medical tomes; they are instead lined with rare physics texts from the early 20th century, including several volumes of the Annalen der Physik, which published the pioneering work of Einstein, Hertz and Planck. Nestled among these is a copy of Everyone Poops—Gatenby recently became a grandfather.
The most curious (and most telling) book on Gatenby’s shelf is The Truth in Small Doses: Why We’re Losing the War on Cancer-and How to Win It. Having survived Hodgkin’s lymphoma as a young man, Clifton Leaf decided to investigate why cancer medicine had made so few advances in recent years. The resulting Fortune article in 2004, as well as his book last year, offers little cause for optimism, with their depiction of a medical culture whose caution has gradually ossified into maddening inertia.
“I like big thinkers,” Leaf told me when I asked him about Gatenby’s work. “He’s a guy who doesn’t get stuck in orthodoxy.” As Leaf writes in his book, the whack-a-gene approach to cancer has its finest success story in Gleevec, which, since its introduction about a decade ago, has proved an adept warrior against chronic myelogenous leukemia. It does exactly what proponents of the genetic approach to cancer hope, seeking out the tyrosine kinase enzymes that drive the growth of the once-deadly blood cancer. Time magazine put Gleevec on its cover in 2001, asking, “This little pill targets cancer cells with uncanny precision. Is it the breakthrough we’ve been waiting for?”
Unlike most solid-tumor cancers, the type of leukemia Gleevec cured is spurred by a single “driver” mutation. Render it inert, as Gleevec does, and the cancer is largely defeated. But few other iterations of the disease are so simple. The closest successor drug is Herceptin, which targets breast cancer tumors with the HER2-positive genetic alteration. Otherwise, targeted therapy has not fulfilled its promise; chasing after errant genes through the body is like trying to catch a school of tuna with a Ziploc bag.
And so Gatenby and his team have taken the opposite tack, going way big and trying to understand all of cancer, instead of just one or two genes. Gatenby told me a story about a cancer conference in Toronto where all attendees were introduced by the name and the molecule they were studying. He chuckles at this myopia, divorced from any greater vision of the Brobdingnagian disease.
‘A Blind, Emotionless Alien’
Gatenby knows that all his equations will mean nothing if they don’t help patients. Ultimately, he will have to convince the very clinicians who frustrate him that his abstractions can have real-world benefits. He needs to not only predict the hurricane but also save the cities in its path.
In 2000, Gatenby went to the University of Arizona and was named the head of radiology at its College of Medicine in 2005. It was here in the desert of Tucson that he had an intellectual conversion. He had been publishing mathematical models of cancer during the past two decades at Fox Chase, but now he began to understand the role that evolution plays in carcinogenesis. The first principles of cancer that he had been trying to find, Gatenby surmised, lay in the Darwinian concept of natural selection.
Gatenby’s insight was brilliantly counterintuitive: Cancer is really, really good at evolution. So damn good that our bodies nourish it, even as it hijacks blood vessels and nutrients. It fools the immune system, nestling so deep within normal tissue that we can’t easily extract it. And then, in what amounts to suicide, it kills the very body in which it has taken root. The writer Christopher Hitchens once described the esophageal cancer that would soon kill him as a “blind, emotionless alien.” But that alien is actually a native son.
Math could provide a map of cancer’s movements; Gatenby now understood that only Darwin could explain why that movement was so hard to arrest. We were unwittingly helping that evolution along, turning all too many cancers into hurricanes. Worst of all, we were doing it in the name of saving lives.
Gatenby was convinced by studying pest management, of all disciplines. By the early 1970s, the agricultural industry had come to realize the limit of synthetic pesticides, which had been famously demonized by Rachel Carson’sSilent Spring in 1962. They were not only potentially harmful to humans but possibly not all that good at protecting crops. If used indiscriminately, chemical agents would indeed kill plenty of insects, but those that survived had a resistance to the toxic substance, which could do nothing against the remaining pests, which were free to breed. This was the evolutionary version of the cliché about how the thing that doesn’t kill you makes you stronger. Our efforts to vanquish the bugs forced evolution’s hand.
A little more than a month after signing the National Cancer Act on December 23, 1971, Nixon addressed Congress on the nation’s environmental challenges. Among the initiatives he introduced was Integrated Pest Management, which promised “judicious use of selective chemical pesticides in combination with nonchemical agents and methods,” like the deployment of natural predators. Instead of trying to kill all bugs, Integrated Pest Management would try to control the population, less concerned with annihilation than watchful containment. There would always be some bugs; the goal was to keep them from spreading, often by using less than the maximum dosage of pesticide.
Infographics by John Grimwade
The War on Cancer sold the American public (and much of the medical establishment) on the idea that cancers must be vanquished entirely, that no stalemate was possible. Thus the endless rounds of chemo a patient faces today, killing good cells with the bad. Gatenby thought that Integrated Pest Management offered a rejoinder to that all-or-nothing mentality. The bug guys had realized what the cancer guys hadn’t: You raze an entire enemy city, and those who remain will be hardened insurgents with a lust to kill. But trim away strategically at the enemy’s forces, and the rest of the population will be kept at bay. Treatment, in other words, may aggravate a cancer’s growth by stripping away the easy-to-kill cells and leaving behind hardened carcinogenic warriors.
“Evolution will win this game,” Gatenby tells me. Malignant cells will eventually evolve beyond the capacity of any drug to hold them at bay. Even the patients of wonder drug Gleevec face an eventual recrudescence of chronic myelogenous leukemia. The question is whether a mathematical understanding of how cancer progresses can lead to treatments that are less prone to aggravating resistant cells into proliferation.
Gatenby came to Moffitt in 2008 to fix the radiology department. Though he continues to do clinical rounds one day each week, his intellectual energies have clearly shifted to the Integrated Mathematical Oncology Department, which has six full-time members and about a dozen postdoctoral and graduate students. They know that many think they are on a quixotic quest. They know that the money is in drug development. They know that too many people are dying. And yet there they are, jovially scrawling equations on a blackboard.
Ravaged by Rambo
So how do you defeat a disease that is constantly evolving a resistance to our weapons?
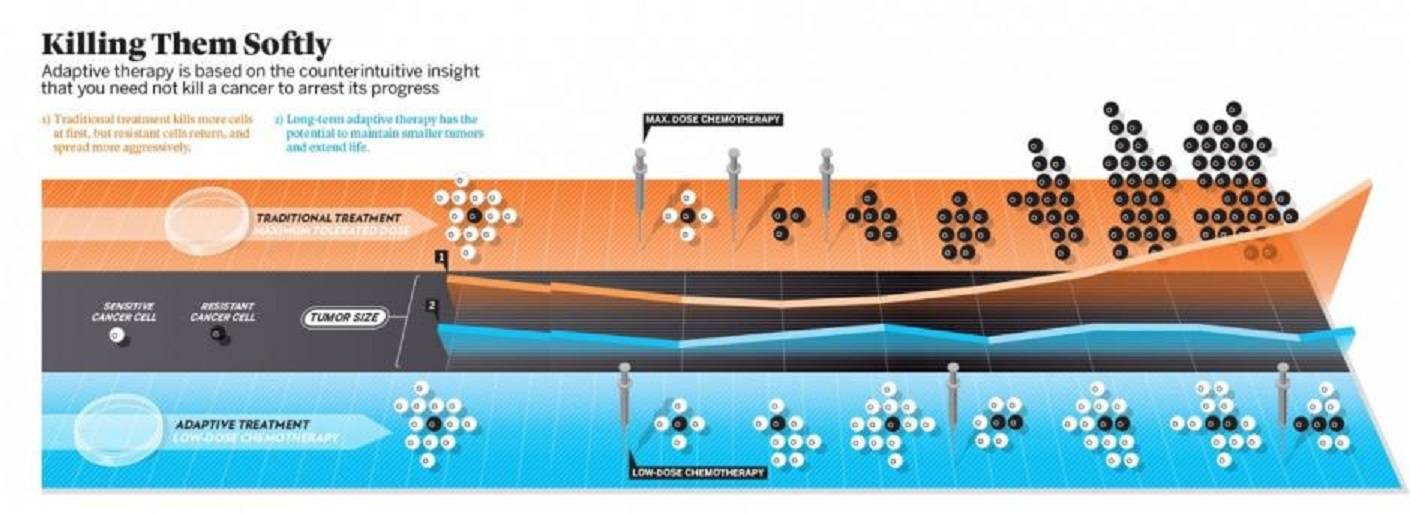
Maybe by turning your swords into plowshares. In 2009, Gatenby published a paper in Cancer Research called “Adaptive Therapy.” Gatenby posited that a tumor consisted, in essence, of chemo-sensitive cells, fast to proliferate, and chemo-resistant cells, more reluctant to grow. The standard practice of giving the maximum tolerated dose of chemo would clear out the sensitive cells, leaving behind a tough nugget of impervious cells, the al-Qaida rebels of the bunch. These previously dormant cells would now pour out of their caves, suddenly finding both space and nourishment to grow. And grow they would, with the barrier of the sensitive cells gone. In essence, cancer therapy was killing “good” cancer cells while leaving behind “bad” ones.
Gatenby used both mathematical models and in vivo experiments to show that adaptive therapy, which kept some of cancer’s petty criminals around, actually held the most dangerous cells in abeyance. One of his co-authors on the 2009 paper was Ariosto S. Silva, a mathematician now at Moffitt. Silva, who is Brazilian, likes to explain cancer through the Rambo film First Blood, about a bloodthirsty Sylvester Stallone protagonist who is made an especially vicious killer because of the suffering he endured in Vietnam. Our approach to chemotherapy is turning cancer cells into Rambos, Silva explained when I met him in Tampa.
A recent innovation is the use of ersatzdroges, or “fake drugs,” which target chemo-resistant cells without killing them. In a draft paper titled “Sweat but No Gain,” Gatenby and his Moffitt colleagues describe how multi-drug-resistant cells “continue to activate their membrane pumps to extrude the ersatzdroge as though it were a cytotoxic agent,” that is, an actual chemotherapeutic drug. But it isn’t. The cells don’t know that, however, working furiously to defend themselves, “thus causing a decrease in fitness by limiting available resources for proliferation and invasion.” While research into ersatzdroges is relatively new, Gatenby’s paper suggests that tiring cells out instead of killing them does slow tumor growth. Instead of evolving with their resistance, the cells expend all their energy staying afloat.
Some oncologists are skeptical of Gatenby’s approach. Robert Weinberg, the MIT cancer researcher who discovered the first cancer-causing gene, does not believe mathematical oncology is a fruitful pathway because it “lacks predictive powers that extend beyond predictions made from simple, intuitive assessments of future behavior,” as he told me in an email. Others say that while Gatenby’s evolutionary portrait of cancer is a clever analogy, it is not instructive for treatment of the disease. Marc B. Garnick, a urologic cancer specialist at the Beth Israel Deaconess Medical Center and Harvard Medical School, says Gatenby’s ideas mirror the practice of metronomic therapy, which “has been around for decades.” (Gatenby disputes this claim.)
But for clinicians frustrated with the current pace of progress in the War on Cancer, Gatenby at the very least offers a new way of thinking about a disease that has perplexed humanity for thousands of years.
Athena Atkipis, an evolutionary biologist and co-founder of the Center for Evolution and Cancer at the University of California, San Francisco (and a sometime colleague of Gatenby’s), likes to think of the different approaches to the War on Cancer in terms of Greek mythology. “My name is Athena, I know,” she says by way of disclaimer before launching into an explanation: The god of war, Ares, understandably “likes a good fight.” Athena, meanwhile, is a master strategian, always scheming about how to outwit the enemy. Her namesake would know better, Atkipis implies, than trying to “intimidate the cancer into retreating.”
In Tampa, Gatenby and I went to dinner at a Cajun restaurant with Anderson and Silva, two of Moffitt’s mathematicians. Downing his first drink, Anderson indicated his distaste for most of what passes for oncology today: “We keep measuring s**t without getting anything out of it.” Everyone laughed. Gatenby complained about how the first fancy car he’d ever purchased had had its cool compromised by the installation of a car seat. Everyone laughed again. It was like the scene in The Untouchables when Eliot Ness, the famed Prohibition enforcer played by Kevin Costner, takes his lawmen out for a celebratory dinner after a huge cache of booze has been found and summarily destroyed. They are drunk with elation, but also a little anxious: The main target, Al Capone, remains at large. So it was in Chicago. So it was in Tampa, only with drinks.
“I’ve never seen a dogma I didn’t hate,” Gatenby told me. That was true at the Catholic school in Pennsylvania. And it is true today, at the hospital in Florida, where the cancer patients are waiting.
(Reprinted with permission from Newsweek)
 The Global Calcuttan Magazine
The Global Calcuttan Magazine